Montréal-based healthtech startup Ditch Labs has acquired a portfolio of patents from Paris, France-based electronic cigarette company Enovap SAS for an undisclosed amount.
The acquisition of Enovap’s entire portfolio of patents, which are valid in 15 countries, adds to Ditch Labs’ intellectual property (IP) holdings. The deal comes nine months after it secured $3.25 million CAD in seed funding, following which Ditch Labs CEO Laurent Laferrière told BetaKit the company’s biggest advantage over its competitors was its IP, referring to the patents it holds for its “dual-tank, dual-coil technology” in 15 countries.
Ditch Labs said this transaction will also help it expand its reach in inhalation technology development and the potential for licensing and strategic partnerships. The company said in a statement that these partnerships will be essential for advancing solutions globally.
RELATED: Ditch Labs secures $3.25 million to treat nicotine addiction with tech
“By integrating these key assets, we are ready to expand our reach and deliver cutting-edge solutions that cater to the unique needs of individuals battling nicotine addiction,” Laferrière said in a statement.
Founded in 2020 by Laferrière, CTO Olivier Bourbonnais, and chief scientific officer Christelle Luce, Ditch Labs aims to help smokers wean themselves off nicotine with its dual hardware-software solution. The company, which has raised $5.7 million to date, has backing from Amplify Capital, Boreal Ventures, Symphony Health Network, Anges Québec, among others.

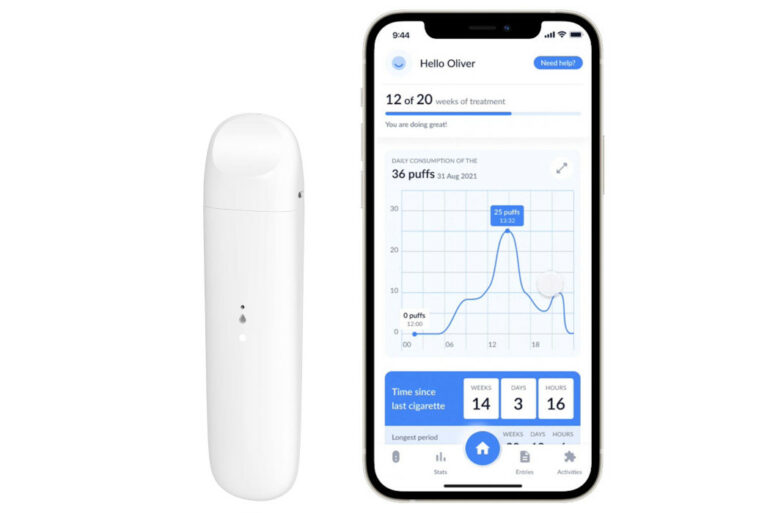
Ditch Labs’ solution consists of a medical nicotine vaporizer that facilitates controlled delivery while an app provides personalized support, progress tracking, and behavioural interventions. Its patented two-tank, two-system vaporizer tech enables it to vary doses and distribute placebos to users.
By combining the hardware-level nicotine moderation and software-level support, the startup aims to address the physiological and psychological impacts of nicotine addiction simultaneously.
Ditch Labs has had several discussions with Health Canada and the US Food and Drug Administration, Laferrière told BetaKit in July, adding that regulatory requirements leave the startup roughly three years away from being able to commercialize its tech in Canada and the US.
In response, Ditch Labs has decided to strategically pivot to the UK market, where Laferrière said it faces a favourable regulatory environment. The company plans to conduct an inaugural clinical trial in 2024 and officially launch its product to the UK market in Spring 2025. Laferrière told BetaKit that pursuing commercialization in other regions would have entailed approximately three more years of regulatory hurdles.
UPDATE (12/11/23): This story was updated to note additional information shared by Ditch Labs CEO Laurent Laferrière.
Feature image courtesy Ditch Labs.